Snow Crystals (2)
Professor LC showed me the snow crystals, which were clearly hexagons. Apparently, there are no pentagons or octagons.
I asked why they are hexagons.
When air that contains water vapor cools at high altitude and becomes supersaturated (which occurs if the temperature drops when the concentration at which water dissolves in air has been exceeded), water molecules centered around extremely fine dust particles condense from the vapor, solidifying to produce grains of ice.
When water molecules clump together, it is due to a mutually attractive force known as the hydrogen bond, which enables growth in both the longitudinal direction and the in-plane direction. When growth occurs in the in-plane direction, the three hydrogen atoms in the vicinity of oxygen become equivalent, resulting in a bond angle of 120 degrees and the creation of a basic hexagonal structure.
What is the Hydrogen Bond?
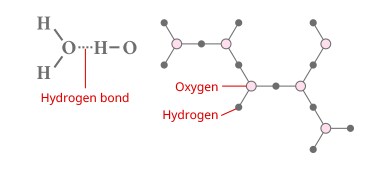
This is the mutually attractive force between hydrogen and the lone pairs of electrons (electrons not participating in ordinary bonds) in oxygen and nitrogen. The reason that liquid water has a high boiling point despite its low molecular weight is that the molecules are weakly attracted to each other by hydrogen bonds. Additionally, the volume expands when liquid water becomes ice because the molecules are arranged at regular intervals due to the influence of the hydrogen bonds, creating small gaps. Incidentally, developing analytical conditions or troubleshooting with a high-performance liquid chromatograph (HPLC) can only be done properly by considering the effect of hydrogen bonds between the target component and the solid phase.
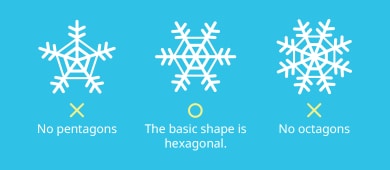
The basic shape of snow crystals is hexagonal!
When the conditions are such that the temperature is about -15 °C and the humidity is 110 % or higher (*), ice grows in the plane and typical arborescent (star-shaped) hexagonal (snow) crystals form. While falling, the water molecules touch at the branches of these crystals, thereby forming even larger crystals. (* The supersaturation for ice)
Note that even when the humidity is the same, if the temperature is about -12 °C or -18 °C, hexagonal plates will form. Additionally, at -8 °C, a needle shape forms, and at -20 °C or below, hexagonal prisms forms, so the shape of the crystal changes depending on the temperature and the humidity.
It is Ukichiro Nakaya who provided a chart of the relationship between the shape of the crystals and the temperature and humidity in the upper air. In his wonderful quote, “Snowflakes are letters sent from heaven,” and points out that these “show us the conditions in the upper atmosphere.”
The snow crystals may continue to grow as they fall but if the air on the way down is warm, the crystals will melt and these “letters” will not be delivered.

Many children incorrectly draw pentagonal or octagonal snow crystals. We would like to clear this up by showing them more scientific observations.
Incidentally, snow appears white to the naked eye, but it is in fact clear and colorless. It is not that it absorbs specific visible light, but rather that it appears white because of reflected light.


