Method Transfer and High-Speed Analysis of Impurities of Drug by Prominence-i
In February 2014, the Japanese Pharmacopoeia - Sixteenth Edition, Supplement Ⅱ was newly issued. Bepotastine besilate1), newly listed among the official monographs (chemicals, etc.), is a selective histamine H1 antagonist which is prescribed for treatment of itching of the eyes, often associated with allergic conjunctivitis. Here, using the new Prominence-i integrated high-performance liquid chromatograph, we conducted analysis of substances chemically related to bepotastine besilate in accordance with the Japanese Pharmacopoeia, while at the same time comparing the analysis results between those using the Prominence-i (with the standard configuration or with the delay volume-compatible system kit) and those obtained using both the conventional integrated LC-2010 model as well as a third-party company's LC system. In addition, we introduce an example of method transfer from that used with Shimadzu's conventional system or that of a third-party LC system, respectively. At the same time, we introduce an example of high-speed analysis using the Prominence-i (with Low-volume tubing kit installed) in which the analysis time is shortened to about one-fourth the time, while conforming to the Japanese Pharmacopoeia default of conditions.
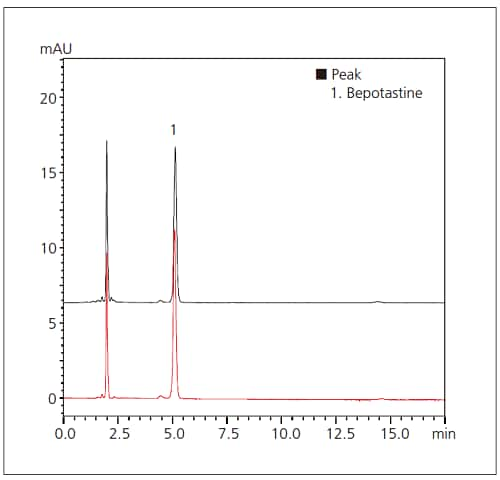
Fig. 1 Chromatograms of Bepotastine Besilate
Upper: Third-Party LC System
Lower: Prominence-i


