Protein Secondary Structural Analysis by FTIR
X-ray diffraction analysis and NMR are widely used for the structural analysis of proteins. However, infrared spectrophotometry is used for the secondary structural analysis, such as α-helices and β-sheets. As infrared spectrophotometry makes it easy to measure samples in all forms (solid/liquid and crystalline/non-crystalline), it is used to complement the analytical methods mentioned above.
Fig. 1 shows the transmission spectrum of bovine serum albumin.
The peak near 1650 cm-1 in Fig. 1 is the amide I band. It results from the C=O stretching vibrations of the peptide bond.
Similarly, the peaks near 1540 cm-1 (N-H bending vibration/C-N stretching vibration) and 1240 cm-1 (C-N stretching vibration/N-H bending vibration) are called the amide II band, and amide III band, respectively. The peak near 3300 cm-1 is thought to be N-H bending vibration and the peak near 1400 cm-1 to result from protein side-chain COO-.
As the absorption peak position and shape of the amide I band differ according to the secondary structure, peak analysis can yield information on the secondary structure.
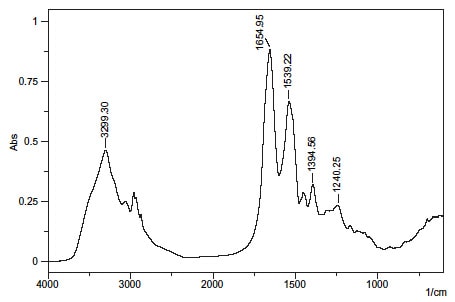
Fig. 1 Transmission Spectrum of Protein (Bovine Serum Albumin)
Infrared Spectrophotometer

An infrared spectrophotometer is mainly used to estimate the structure of organic compounds (qualification).
This instrument shines infrared light onto the molecules, which absorb infrared radiation equivalent to the interatomic vibrational energy of the atoms that comprise the molecules. It then estimates the structure and quantifies the compound by investigating this IR absorbance.


