
Nexis GC-2030

Industria
Clean Energy
PALABRA CLAVE
INTRODUCCIÓN SERVICIO Y PRODUCTOS
Nexis GC-2030, Lightway
We visited Professor Kyung Byung Yoon at Sogang University in Korea. He is one of the leading researchers in the field of artificial synthesis. His laboratory features many analytical instruments, including the Tracera GC-BID system and the QYM-01 photoreaction quantum yield evaluation system*, which permits accurate and easy simple quantitation measurements of absorbed photons.
* The QYM-01 system is not available in Europe and may not be available in some other countries. Please contact your local Shimadzu representative for availability.

*Los afiliados y los títulos del entrevistado están actualizados al momento del informe.
Sogang University
URL
http://wwwe.sogang.ac.kr/
Would you please introduce your research?
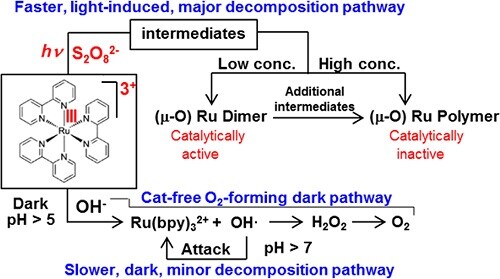
The most widely accepted system for homogeneous photocatalytic water oxidation process consists of a water oxidation catalyst, RuII(bpy)32+ as a photopump, and S2O82− as the sacrificial electron acceptor. However, this system is far less than ideal because RuII(bpy)32+ undergoes very rapid decomposition and as a result the process stops before all of the S2O82− is consumed. In this regard its decomposition pathways and the fate of RuII(bpy)32+ should be elucidated to design more efficient photocatalytic water oxidation systems.
We found that two pathways exist for decomposition of RuII(bpy)32+ in the light−RuII(bpy)32+−S2O82− system. The first is the formation of OH• radicals at pH >6 through oxidation of OH‑ by RuIII(bpy)33+ in the dark, which attack the bpy ligand of RuII(bpy)32+. This is a minor, dark decomposition pathway. During irradiation not only RuII(bpy)32+ but also RuIII(bpy)33+ becomes photoexcited and the photoexcited RuIII(bpy)33+ reacts with S2O82− to produce an intermediate which decomposes into catalytically active Ru μ-oxo dimers when the intermediate concentration is low or into catalytically inactive oligomeric Ru μ-oxo species when the intermediate concentration is high. This is the major, light-induced decomposition pathway. When the RuII(bpy)32+ concentration is low, the light−RuII(bpy)32+−S2O82− system produces O2 even in the absence of any added catalysts through the O2-producing dark pathway. When the RuII(bpy)32+ concentration is high, the system does not produce O2 because the overall rate for the light-induced decomposition pathway is much faster than that of the O2-producing dark pathway (ACS Catal.2016,6, 8361−8369).

Are "QYM-01" and "Tracera (GC+BID Detector)" being efficiently used for your research?
How are they useful?
QYM-01 can take a UV-Vis spectrum every minute or shorter than that. This allows us to monitor how fast a material reacts during photoreaction. Also, it can measure the number of photons that a photosensitizer absorbs. When we detect products, the quantum yield of the reaction is calculated by plotting the number of absorbed photons versus generated products.
Tracera is efficient for checking liquid products. The detection sensitivity was also good. Almost all materials were detected in the column.
What do you think about the advantages and benefits of "QYM-01" and "Tracera (GC+BID Detector)"?
We can take a UV-Vis spectrum during photolysis without changing any other reaction system. We can measure the power of light we are using. This is the advantage of QYM-01. As for Tracera, the detection limit is good.
Do you have any suggestions about improving or enhancing the features/functionality of the "QYM-01" and "Tracera(GC+BID Detector)"?
Firstly, the intensity of light that reaches the reaction site needs to be higher. At a wavelength, the power of light was 1 to 2 mW/cm2. Secondly, the gas inlet and outlet from the QYM-01 needs to be installed like ports for water circulation. Lastly, to check the power of light, we have to remove the lid of the machine and move the detector.
As for Tracera, the detection of formic acid needs many steps.
Do you have any requests or expectations for Shimadzu technology, products and service?
The stirring system has a lifetime. So, I hope to replace it immediately when it stops.
Please let us know your image of "Shimadzu Corporation"
You are providing an unprecedented product and good service.
It was great time to talk to you and to know what you think about our instruments and our attitudes. We must try to keep getting better. Thank you very much.



Tracera High Sensitivity
Gas Chromatograph System
After the interview, Professor Yoon said, "Because of few examples of its installation, QYM has a lot to be improved, such as stability or usability. On the other hand, I am impressed that Shimadzu has an original unique technology without precedent, such as QYM, and look forward to its future advancement." What pleased us more was that he added, "We can cooperate together to be the bridge of Korea and Japan, and to make both countries more affluent through Shimadzu instruments and solutions."